Anion vs. cation: What they are, a chart, and the periodic table
If there are an equal number of electrons (negative charge) and protons (positive charge) in an atom or atoms, they are neutral. They will be charged, though, if they are not fair. Ions are the name for these charged particles.
What’s the difference between cations and anions?
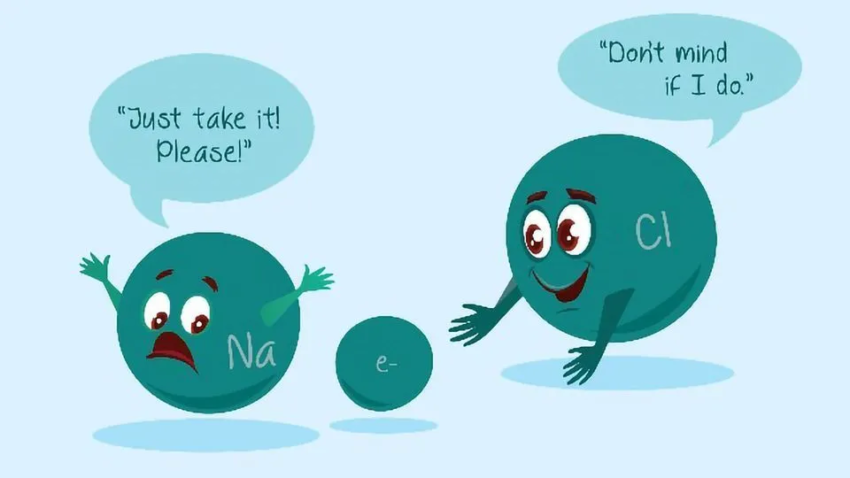
Ions that are positively charged are called cations. It is an ion that has a negative charge. Ions are atoms or molecules that are charged. One or more electrons must be taken away from a balanced atom for it to become a positively charged cation. It is called an anion if an atom that is balanced adds one or more electrons.
What does a cation do?
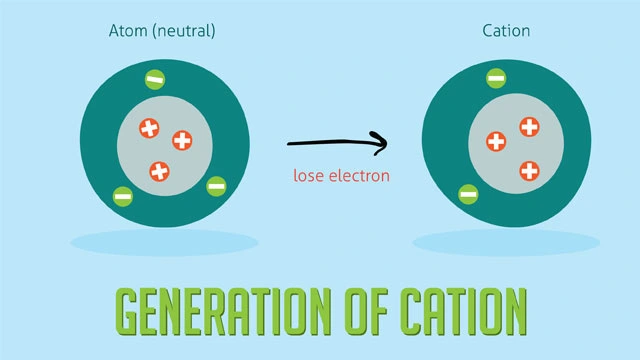
A cation has a net positive charge because it has more protons than electrons. For a cation to form, one or more electrons must be lost. Atoms that are more attracted to those electrons tend to pull them away. It shows how many electrons the ion lost, which determines its charge. For example, silver (Ag) loses one electron to become Ag+, while zinc (Zn) loses two electrons to become Zn2+.
It is shown how the energy of an atom changes when it turns into a cation.
The process of making a charge.
What is an anion?
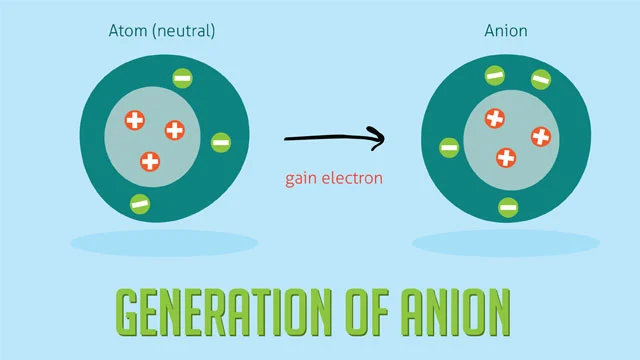
An anion has a net negative charge because it has more electrons than protons. One or more electrons must be won, which usually means pulling them away from atoms that don’t stick to them as well. It says how many electrons the ion gained, which tells you its charge. For example, chlorine (Cl) gains one electron to become Cl-, and oxygen (O) gets two electrons to become O2-.
It is shown how the energy of an atom changes when it turns into a cation.
The process of making an anion.
Acid vs. Base Chart
The table below shows the main ways that cations and anions are different.
| Cation | Anion | |
| Charge | Positive | Negative |
| Electrode attracted to | Cathode (negative) | Anode (positive) |
| Formed by | Metal atoms | Non-metal atoms |
| Examples | Sodium (Na+), Iron (Fe2+), Ammonium (NH4+) | Chloride (Cl–), Bromide (Br–), Sulfate (SO42-) |
Some of the electrons in metallic atoms are held on to pretty lightly. Because of this, they tend to lose electrons and turn into cations. Most nonmetallic atoms, on the other hand, draw electrons more strongly than metallic atoms, which means they gain electrons and turn into anions. So, when atoms from a metallic element and a nonmetallic element come together, the nonmetallic atoms tend to take one or more electrons away from the metallic atoms, which makes ions. The charged ions then stick together to make ionic bonds, which create ionic molecules with no net charge overall. Some examples are magnesium oxide (MgO), potassium iodide (KI), and calcium chloride (CaCl2).
Table of cations and anion
Based on where an atom is on the periodic table, you might be able to guess whether it will make a cation or an anion. Metals that are alkaline or earthy always make cations, while metals that are halogen or acidic always make anions. Other metals, like iron, silver, and nickel, tend to form cations, while most nonmetals, like oxygen, carbon, and sulphur, tend to form anions. On the other hand, some elements can become both cations and anions if the circumstances are right. Like hydrogen, which can either gain (H-) or lose (H+) an electron, it can make hydride compounds like ZnH2 (where it is an anion) and hydron compounds like H2O (where it is a cation).
The “noble gases” in group 18 of the periodic table don’t usually combine with other things because of the way their electrons are arranged. This means they don’t form ions.
The table of elements.This is the periodic table
Size of cations vs. anion
Over the whole table, cations and anions come in a lot of different shapes.
Using the features of ions
Chemists can use ionic qualities for a number of different tasks. In ion-exchange chromatography, for example, the molecules being separated stick to the stationary phase because of the way their charges are structured. This makes separation possible.
Ionic qualities are also very important to how batteries work. There are two plates in a battery. The cathode is the positive end, where the electricity leaves and the electrons enter, and the anode is the negative end, where the electricity enters and the electrons leave. There is an electrolyte liquid or gel between the electrodes. It has charged particles called ions in it. An electric current is made when this ionic substance mixes with the electrodes. Zinc is often used as the anode in single-use dry cell batteries, and manganese dioxide is often used as the liquid cathode. In zinc-carbon batteries, the zinc anode is also the battery’s case. As it oxidises during use, the contents can start to leak over time.
On the left is a zinc-carbon dry cell battery, and on the right is an alkaline battery.
A zinc-carbon dry cell battery is on the left, and an alkaline battery is on the right.
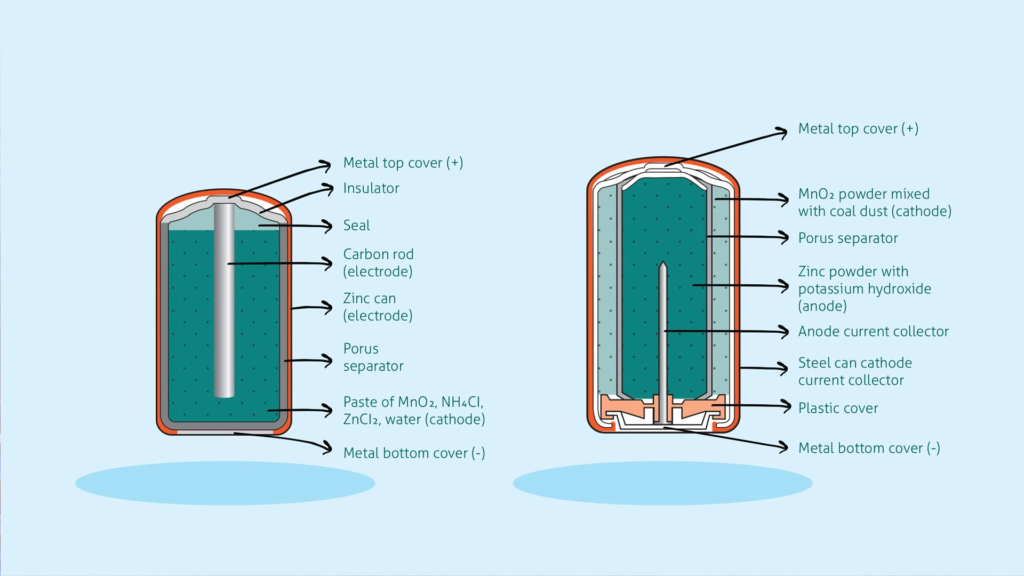
This chemical process can be turned around in rechargeable batteries, like many lithium-ion batteries, and the internal structure changes. This lets the batteries be charged again and again.
Because salt water has ionic qualities, scientists are now trying to find ways to use salinity gradients, which are places where salt water and fresh water mix, to make ionic electricity as a green source of energy for the future.