Chart with Abbreviations and Structure of Essential Amino Acids
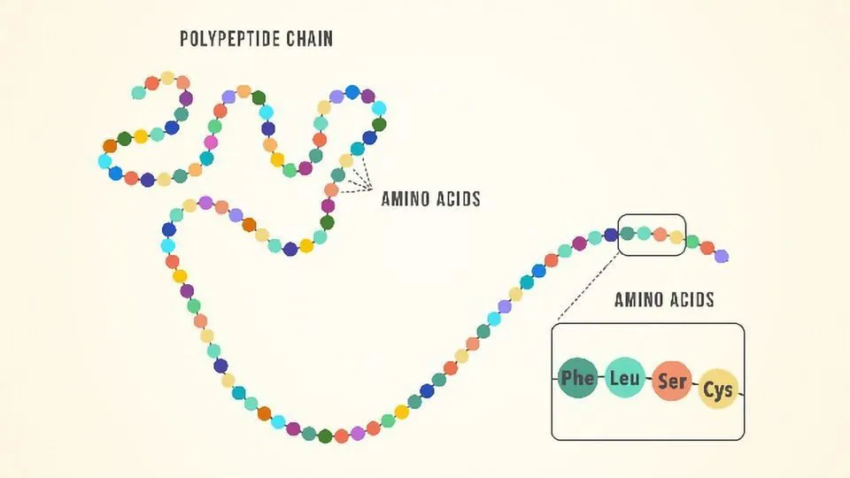
Amino acids are the constituents of polypeptides, which are proteins. They are therefore fundamental components of our bodies and essential for functions including protein synthesis, tissue repair, and nutrient absorption. Amino acids are derived from and function in the body in more detail here.
List of amino acids
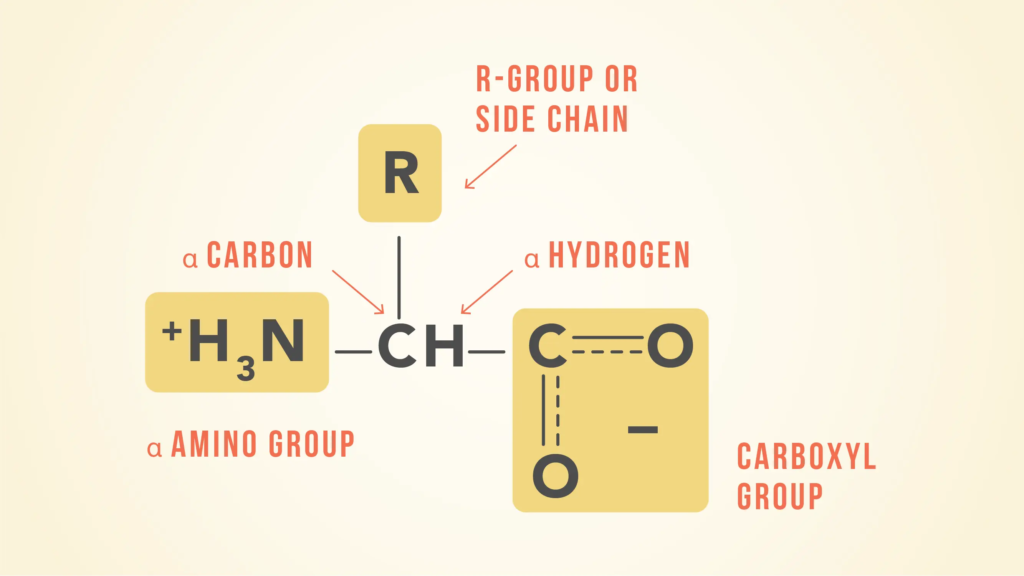
Twenty amino acids comprise proteins. Their R-groups or side chains are the sole differences between them; otherwise, they all have the same basic structure.
As may be seen below, the proton (H+) alternates between the amino and carboxyl groups. This keeps the amino acids in balance.
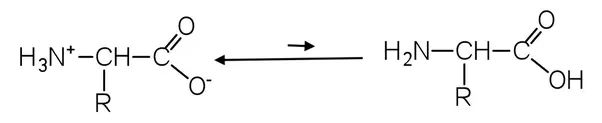
In this kind of balance, the weaker acid always wins. It will be on the left (the “zwitterion” side) because ammonia is not as strong of an acid as carboxylic acid. In texts, amino acids are often shown as having a right-hand structure, but in real life, they mostly have a left-hand structure.
When it comes to size and complexity, glycine is the simplest amino acid. Its R-group is a hydrogen (H).Smaller groups can be formed from them according to the characteristics that their functional groups define. The three main categories into which they fall are charge, hydrophobicity, and polarity. The way these elements interact with adjacent amino acids in polypeptides and proteins alters the three-dimensional shape and characteristics of the proteins.
Scientists have figured out how the 20 amino acids that make up proteins are put together chemically.
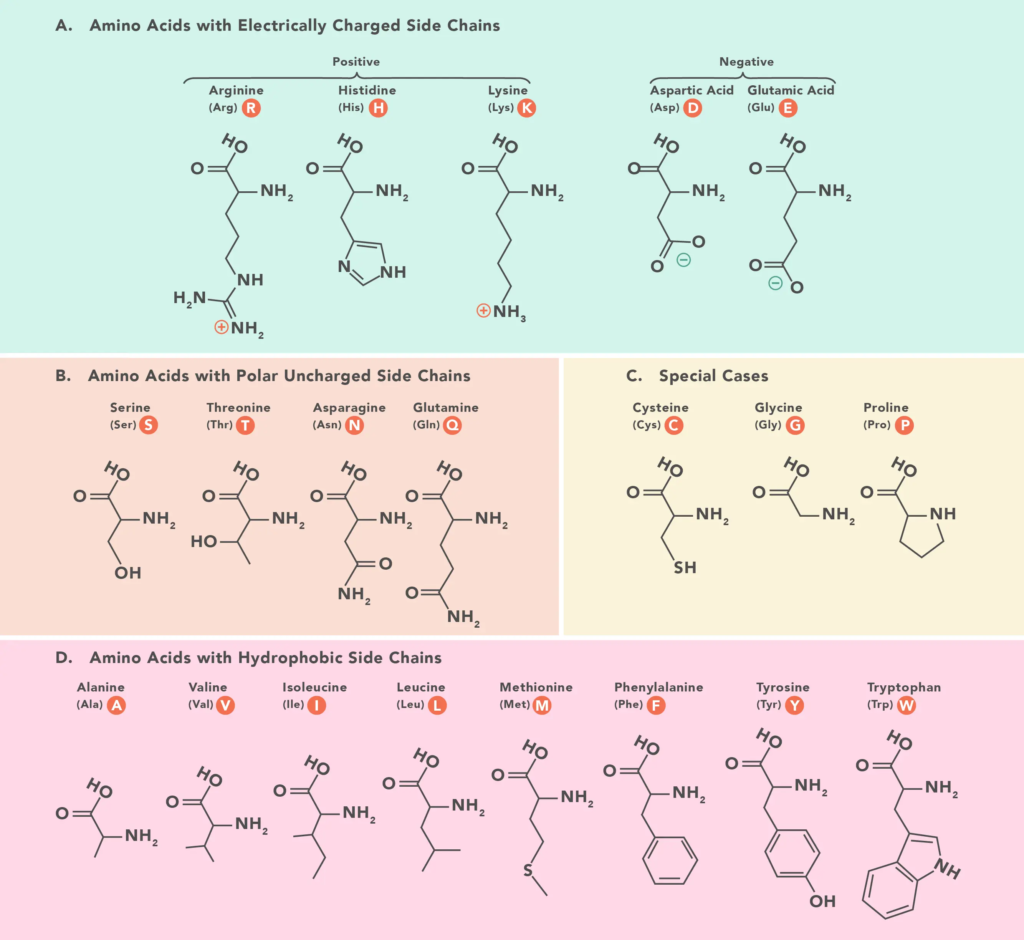
This graph shows how the 20 amino acids that make up proteins are put together chemically.
Shortcuts for amino acids
The single letter names and acronyms for the 20 amino acids that comprise proteins are shown in this table. Blue also includes selenocysteine, an equivalent of cysteine found only in some lineages, and pyrrolysine, which is used to create proteins in some archaea and bacteria but not in humans. To finish off the alphabet of single letter abbreviations, the ending codon and amino acid residues that can have more than one possible name are shown in red.
How are the amino acids put together?
There is a carbon atom in the middle that is connected to a hydrogen atom, an acidic carboxyl group (−COOH), an amino group (−NH2), and an organic side chain (also called a R group). Each of the 20 amino acids has a different side chain.
| Amino acid | Abbreviation | Single letter abbreviation |
| Alanine | Ala | A |
| Arginine | Arg | R |
| Asparagine | Asn | N |
| Aspartic acid | Asp | D |
| Cysteine | Cys | C |
| Glutamine | Gln | Q |
| Glutamic acid | Glu | E |
| Glycine | Gly | G |
| Histidine | His | H |
| Isoleucine | Ile | I |
| Leucine | Leu | L |
| Lysine | Lys | K |
| Methionine | Met | M |
| Phenylalanine | Phe | F |
| Proline | Pro | P |
| Serine | Ser | S |
| Threonine | Thr | T |
| Tryptophan | Trp | W |
| Tyrosine | Tyr | Y |
| Valine | Val | V |
| Pyrrolysine | Pyl | O |
| Selenocysteine | Sec | U |
| Aspartic acid or Asparagine | Asx | B |
| Glutamic acid or Glutamine | Glx | Z |
| Any amino acid | Xaa | X |
| Leucine or Isoleucine | Xle | J |
| Termination codon | TERM |
Ala is an amino acid
Alanine was first found in protein in 1875, and it makes up 30% of the residues in silk. Silk’s simple, long structure with few cross-links gives the fibres strength, stretch resistance, and flexibility. Its low reactivity helps make this possible. In the process of making proteins, only the l-stereoisomer is involved.
Arg acid
Arginine is made in people when proteins are broken down. The body can then change it into nitric oxide, a gas that is known to make blood vessels relax.
Because it widens blood vessels, arginine has been suggested as a way to help people with chronic heart failure, high cholesterol, poor circulation, and high blood pressure, though more study needs to be done in these areas. Synthetic arginine can also be made, and compounds similar to arginine can be used to treat people whose livers aren’t working right because they help the liver grow back. Arginine is needed for growth but not for maintenance, but study has shown that it is very important for wound healing, especially in people who don’t have good circulation.
An amino acid
In 1806, asparagine was separated from asparagus juice. This was the first time an amino acid was separated from a natural source. Scientists were able to show that asparagine was found in proteins in 1932 for the first time. It is only the l-stereoisomer that is involved in the production of proteins in mammals. Asparagine is a key part of the body’s process of getting rid of harmful ammonia.
Asp is an amino acid.
Aspartic acid was first found in proteins in 1868. It is mostly found in animal proteins, but only the l-stereoisomer is involved in making proteins. This amino acid is soluble in water, which makes it a good fit for being close to enzymes that do work, like pepsin.
Cys is an amino acid.
Cysteine is found in large amounts in the proteins of hair, hooves, and skin’s keratin. It was first isolated from a urinary calculus in 1810 and from horn in 1899. After that, it was made chemically, and in 1903–4 the structure was figured out.
The thiol group with sulphur in cysteine’s side chain is an important part of its qualities. It lets disulfide bridges form between two peptide chains (like insulin) or loops form within a single chain, which changes the structure of the protein as a whole. Cysteine is an amino acid that is made up of two cysteine molecules connected by a disulfide bond. It is sometimes mentioned separately in lists of amino acids. This amino acid is made in the body from serine and methionine, and it is only found in human proteins as the l-stereoisomer.
People who have the genetic disorder cystinuria can’t get cystine back into their system properly. Because of this, their pee has a lot of cystine, which crystallises and forms stones that block their kidneys and bladder.
Gln acidic amino
In 1883, glutamine was first separated from beetroot juice. In 1932, it was separated from a protein, and the next year, it was chemically made. Glutamine is the amino acid that our bodies make the most of and it has many important jobs to do. In people, glutamine is made from glutamic acid. This process is very important for controlling the amount of harmful ammonia in the body and making urea and purines.
Amino acid glu
In 1866, glutamic acid was separated from wheat gluten. In 1890, it was chemically made. It is usually found in animal proteins, but only the l-stereoisomer is found in mammalian proteins. People can make these proteins from the common intermediate α-ketoglutaric acid. Monosodium glutamate (MSG), which is the monosodium salt of l-glutamic acid, is often used as a seasoning and to make food taste better. The carboxyl side chain of glutamic acid can donate and take ammonia, which is harmful to the body. This lets ammonia get to the liver, where it is changed into urea, and then the kidneys get rid of it. Free glutamic acid can also be changed into sugars or broken down into carbon dioxide and water.
Glycerol is an amino acid.
Glycine was the first amino acid to be separated from a protein, in this case gelatin. It is also the only amino acid that doesn’t have any d- or l-stereoisomers, which means it isn’t optically active. It is the most basic of the α-amino acids and doesn’t react much with proteins when added. Even so, glycine is necessary for making purines, heme, the amino acid serine, and the coenzyme glutathione. Heme is an important part of haemoglobin.
This amino acid
Histidine was first found on its own in 1896, and in 1911, chemical synthesis confirmed its structure. Histidine is the direct building block of histamine. It is also a key carbon source in the production of purines. Histidine’s side chain can take and give protons when it is part of proteins. This gives enzymes like chymotrypsin and those that break down carbohydrates, proteins, and nucleic acids important properties.
Histidine is an essential amino acid for babies. Adults can go for short times without eating it, but it is still considered essential.
Ile is an amino acid
Isoleucine was first found in molasses made from beet sugar in 1904. The fact that isoleucine’s side chain doesn’t like water plays a big role in figuring out the third structure of proteins that contain it.
People who have maple syrup urine disease, a rare inherited problem, have an enzyme that doesn’t work right in the pathway that breaks down isoleucine, leucine, and valine. Metabolites build up in patients’ urine if they don’t get treatment, giving it the unique smell that gives the disease its name.
Leu is an amino acid.
In 1819, leucine was found in cheese. In 1820, it was found in muscle and wool that was crystallised. Synthesis of it took place in a lab in 1891.
Only the l-stereoisomer is found in mammalian proteins, and the body’s enzymes can break it down into simpler molecules. There are parts of some DNA-binding proteins where leucines are grouped in what are known as “leucine zippers.”
Lys is an amino acid.
It was first separated from the milk protein casein in 1889, and in 1902, its composition was fully understood. An important part of how enzymes and coenzymes work is lysine. Histones also work in a critical way.
A lot of cereal crops don’t have much lysine, which can make people who depend on them for food, like vegetarians and people on low-fat diets, get sick. Because of this, work has been done to create maize types that are high in lysine.
Met acid
In 1922, methionine was separated from the milk protein casein. In 1928, its structure was figured out by making it in a lab. Methionine is a key source of sulphur for many molecules in the body, such as taurine and cysteine. Because it contains sulphur, methionine helps keep the liver from storing fat and gets rid of cellular waste and toxins.
Methionine is the only essential amino acid that isn’t found in large amounts in soybeans. Because of this, it is made in factories and added to many soy meal goods.
Phe is an amino acid.
In 1879, phenylalanine was first isolated from a natural source: lupine sprouts. In 1882, it was chemically made. In most people, the body can change phenylalanine into tyrosine. But people who have the inherited disease phenylketonuria (PKU), the enzyme that does this conversion doesn’t work. Phenylalanine builds up in the blood if it is not treated, which slows down children’s mental growth. One in 10,000 children are born with the disease. Eating less phenylalanine from a young age can help ease the symptoms.
A pro amino acid
Proline was scientifically made in the year 1900. It was then separated from the milk protein casein the next year, and its structure was found to be the same. Proline is only found as the l-stereoisomer in animal proteins, but humans can make it from glutamic acid. When added to proteins, proline’s unique structure causes sharp bends, or kinks, in the peptide chain, which is a big part of how the protein is finally structured. Proline and its derivative hydroxyproline make up 21% of the amino acid residues in collagen, which is an important protein for joint tissue.
Ser is an amino acid
Serine was first taken out of silk protein in 1865, but its structure wasn’t figured out until 1902. People can make serine from other chemicals, like glycine, but proteins from mammals only contain the l-stereoisomer. There are many metabolites that depend on serine for their production, and enzymes like chymotrypsin and trypsin often depend on it for their catalytic function.
Nerve gases and some poisons work by joining with a serine residue in acetylcholine esterase’s active site and stopping the enzyme from working at all. Acetylcholine is a neurotransmitter that needs to be broken down by esterase activity. If it is not, dangerously high levels will build up and quickly cause seizures and death.
The third amino acid
In 1935, threonine was separated from fibrin and made in a lab the same year. The l-stereoisomer is the only one that shows up in mammalian proteins, and it doesn’t mix with much else. It plays a big part in many reactions in bacteria, but no one knows what role it plays in the metabolism of higher animals, like people.
Trp is an amino acid.
Tryptophan was first isolated from casein (a protein found in milk) in 1901. Its structure was figured out in 1907, but only the l-stereoisomer is found in proteins from mammals. Bacteria in the gut break down the tryptophan in food, creating chemicals like skatole and indole that give poop its bad smell. Vitamin B3 (also known as nicotinic acid or niacin) is made from tryptophan, but not quickly enough to keep us healthy. As a result, we also need to take vitamin B3. If we don’t, we can get a condition called pellagra.
Tyr is an amino acid
Casein, a protein found in cheese, was broken down in 1846 and tyrosine was extracted. It was then made in the lab and its structure was figured out in 1883. People can make tyrosine from phenylalanine, which is only found in animal proteins as the l-stereoisomer. Tyrosine is a key building block for the hormones epinephrine and norepinephrine in the adrenal glands, thyroid hormones like thyroxine, and melanin, a pigment found in hair and skin. Tyrosine residues are often linked to active sites in enzymes. Changing these sites can change the enzyme’s selectivity or stop it from working at all.
People with the dangerous genetic disorder phenylketonuria (PKU) can’t change phenylalanine to tyrosine. People with alkaptonuria, on the other hand, have problems with tyrosine metabolism, which makes their urine look different and turn darker when exposed to air.
Val acidic
After being separated from albumin for the first time in 1879, the structure of valine was proven in 1906. In human proteins, only the l-stereoisomer is found. In healthy people, valine can be broken down into simpler compounds. But in people with maple syrup urine disease, a rare genetic condition, a bad enzyme stops this process, which can be fatal if not addressed.
What the carboxyl group is made of
- There is an amino group and a carboxyl group in every amino acid.
- A peptide bond is formed between the carboxyl group of one amino acid and the amino group of the next amino acid during amino acid polymerization. A water molecule is lost in the process.
Things about amino acids that don’t bind water
- Some amino acids that don’t like water are alanine, valine, isoleucine, leucine, methionine, phenylalanine, tryptophan, and tyrosine.
- Because of how they are categorised, the side chains tend to be pushed away from water. This changes where these amino acids are located in the protein’s third structure.
Things that polar amino acids can do
- Polar amino acid groups are usually found on the outside of a protein after it has been polymerized. This is because the side chain is hydrophilic.
- There are four amino acids that are neutral but not charged. They are aspartate, glutamine, serine, and threonine.
What aromatic amino acids are made of
- There are other types of amino acids besides phenylalanine, tyrosine, and tryptophan, but they all have aromatic side chains.
- So, they all absorb ultraviolet light to different degrees. Tyrosine absorbs the most, while phenylalanine absorbs the least.
What protein production is and how amino acids work
A protein is made up of many amino acids linked together by a peptide bond that goes from the N-terminus to the C-terminus.
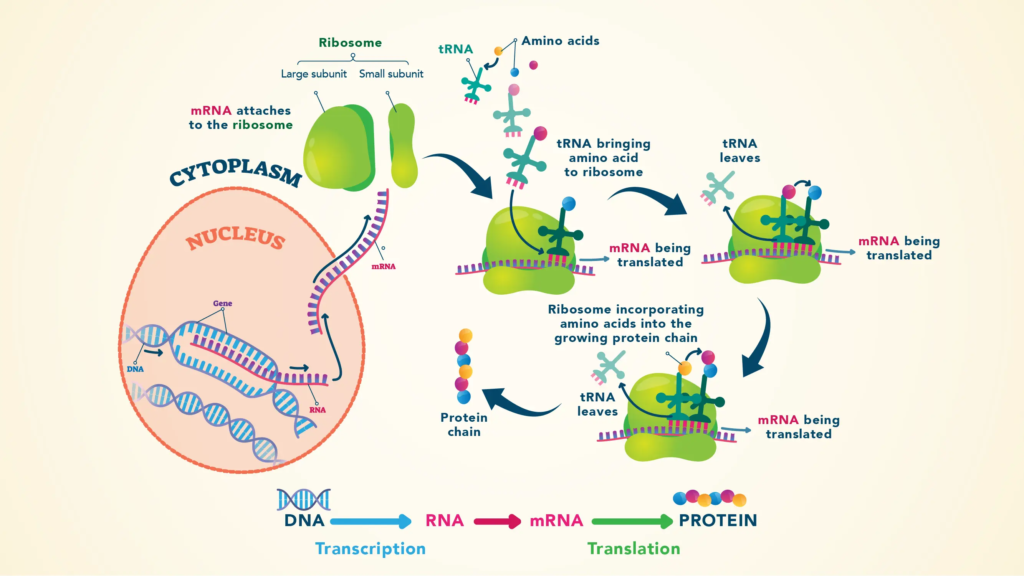
- There is a messenger RNA (mRNA) that is copied from DNA and tells cells which amino acid to put where in order to make a certain protein.
- At the ribosome, a transfer RNA (tRNA) binds to one end of the mRNA and sends the amino acids that are needed at the other end.
- Protein production starts, continues, and ends with the help of other protein factors.
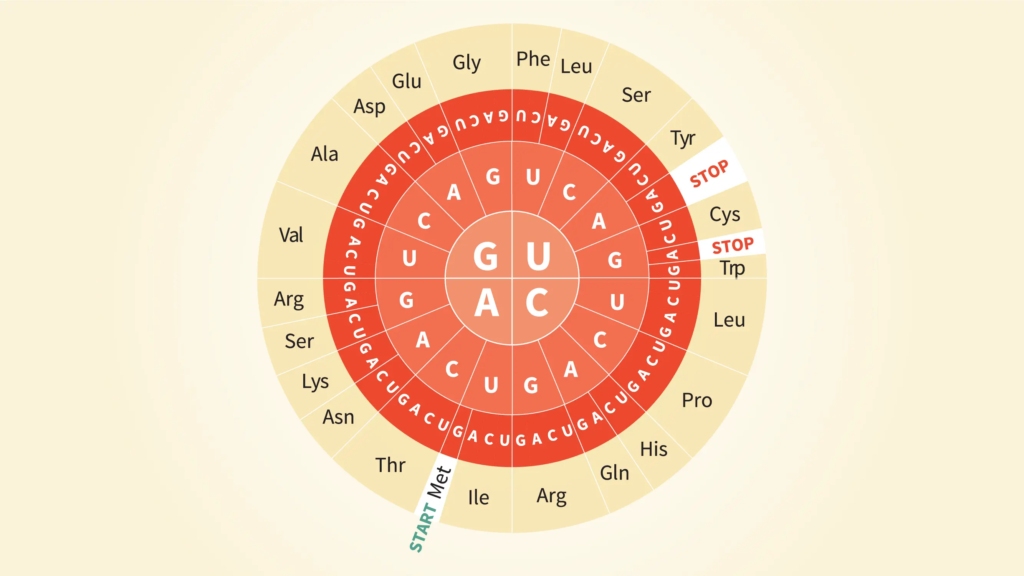
- In the mRNA, which is also known as the “triplet code,” genetic information about which amino acid needs to be added at which place is stored as a set of three bases, or “triplets.” It is made up of 64 possible pairs and the amino acids that go with them. This is known as the structural or amino acid code.
- A lot of the amino acids are coded by more than one triplet. For example, arginine is added when it finds CGU, CGC, CGA, or CGG. Three (or sometimes two) of the triplets signal chain ending happen in most living things.
Nine important amino acids and vitamins for them
Eleven of the twenty amino acids can be made by the body itself, but not the other nine. This is probably because genes are lost or changed over time in reaction to changes in selective pressures, like how much of a certain food there is that contains a certain amino acid. So, we need to get these amino acids from our food, which is why they are called “essential.”
And because different animal types can make different amino acids, their food needs are also different. People, for example, can make their own arginine, but dogs and cats can’t; they have to get it from their food. Unlike people and dogs, cats can’t make their own taurine. One reason why commercial cat food is not good for cats is because of this. The nine amino acids that people need to get from their food are valine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, and tryptophan.
People eat “complete proteins” like meat, seafood, eggs, dairy products, soy, quinoa, and buckwheat because they have all nine necessary amino acids. Some protein sources, like nuts, seeds, grains, and beans, don’t have all of the necessary amino acids, so they are called “incomplete.”
The US suggestion for daily amounts of the nine necessary amino acids is shown in this table. Each amount is based on 1 kg of body weight.
| Amino acid | Recommended daily allowances (mg/kg body weight) |
| Histidine | 14 |
| Isoleucine | 19 |
| Leucine | 42 |
| Lysine | 38 |
| Methionine | 19 |
| Phenylalanine | 33 |
| Threonine | 20 |
| Tryptophan | 5 |
| Valine | 24 |
There are also rules for the amino acids tyrosine (33 mg/kg) and cysteine (19 mg/kg), which are not necessary.
Let’s talk about extras. You can get all the important amino acids your body needs from a healthy, well-balanced diet. Some people, though, say that taking high concentration supplements can help with things like mood, sleep, exercise ability, weight loss, and keeping muscles from losing mass. There are a lot of “health and wellbeing” pages where people are selling amino acid supplements. But is there good evidence to back this up?
Neurotransmitter serotonin plays a big part in sleep, mood, and behaviour. It can’t be made without the vital amino acid tryptophan. Because of this, many studies have looked into what happens when tryptophan amounts are changed and how that affects sleep and mood. There is proof that low levels of tryptophan can make it harder to sleep and make you feel bad, but many studies are flawed in some way, such as having small sample sizes or not having enough controls. So, while it is clear that tryptophan is an important part of a healthy diet and there is a chance that taking a supplement could be helpful, there is currently not enough evidence to support giving tryptophan in amounts higher than what can be found in a healthy diet. More research is needed.
Some studies show that taking amino acid supplements can help some groups do better in exercise. However, the results are very different from one study to the next, and many studies show little to no benefit. A different study is also looking at what happens to skin photoaging when you take an amino acid supplement, but the results have not been released yet.